|
The role of sialylation in the nervous system |
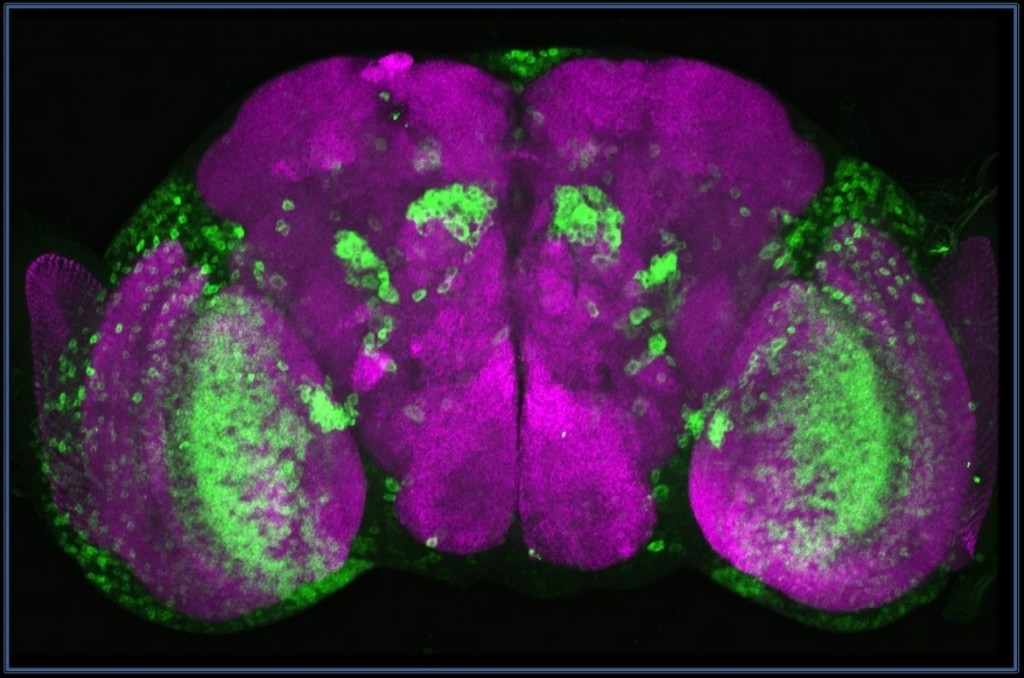 |
Sialyltransferase (green) is expressed in neurons of optic lobes, medulla cortex (MC) and lobula plate (LP), the olfactory system (projection neurons,PN). Frontal projection of a 3D-reconstructed brain from confocal sections. Neuropil is purple (nc82). |
Sialylation influences numerous important biological processes while its defects cause severe pathologies in mammalian organisms. However, until recently, little was known about sialylation in simpler animals, such as protostomes, including Drosophila and other insects. Our laboratory identified and characterized Drosophila sialyltransferase, DSiaT, the first sialyltransferase found in protostomes. Phylogenetic analysis and functional assays revealed that DSiaT is closely related to its mammalian counterparts, ST6Gal sialyltransferases. Unlike mammals that possess 20 different sialyltransferases, Drosophila has only one one sialyltransferase, which makes fruit flies an excellent model system for studying sialylation. The expression of DSiaT is dynamic and limited to subsets of CNS neurons during development and in adults. The expression of CMP-sialic acid synthetase (CSAS), an enzyme generating the CMP-sialic acid sugar donor for DSiaT, is also restricted. Thus, sialylation is tightly controlled in a cell-specific anner, which partially explains why the overall amount of sialylated glycans is very low in Drosophila (see below).
Sialylated glycans represent less than 0.1% of the total glycan profile of the Drosophila N-glycome. Because of low amount, sialylated structures can be unambiguously analyzed only by highly sensitive glycomic approaches, such as multidimensional mass spectrometry. In collaboration with experts in mass spectrometry of glycoconjugates, we characterized N-linked glycans and glycoproteins expressed in Drosophila head and found that sialylated hybrid structures represent the most common type of sialylated glycans in Drosophila. A large dataset of glycopeptide sequences obtained in these experiments also allowed us to analyze sequence preferences for in vivo utilization of N-glycan sequons.
Albeit sialylation is so scarce, it plays a prominent role in the nervous system of Drosophila. The biological function of sialylation was revealed by analyses of DSiaT and CSAS phenotypes. We generated DSiaT and CSAS mutants and analyzing them by behavioral and neurophysiological assays. Genetic inactivation of the sialylation pathway in vivo revealed that sialylated N-glycans play prominent specific roles in the regulation of the nervous system. Sialylation mutants have significantly reduced life span, locomotor abnormalities and temperature-sensitive (TS) paralysis. We found that sialylation affects synaptic growth, regulates neural transmission and controls the function of voltage-gated Na+ channels. Neural excitability can be upregulated or downregulated by manipulation of the sialylation pathway, indicating that sialylated glycans can control the level of excitability of neural circuits. This function is expected to be conserved in humans and represents one of the most evolutionarily ancient roles of sialic acids in metazoan organisms.
Our more recent studies were focused on Drosophila CSAS and its evolutionary relationship with counterparts in other organisms. Unexpectedly, unlike mammalian homologues that function in the nucleus, Drosophila CSAS is a glycoprotein localized in the secretory compartment. This unveils an unprecedented example of radical evolutionary change in subcellular localization of a metazoan enzyme with highly conserved biological functions. The pH and ionic environments of the Golgi and nucleus differ significantly, suggesting that the enzyme underwent evolutionary adaptation to a distinct molecular environment. Our analyses confirmed this hypothesis, revealing several unique features of DmCSAS. They include preference for lower pH and ability to utilize a broad range of metal cofactors. Insect CSAS enzymes evolved a different way of metal coordination within their active sites. In vitro assays demonstrated that Drosophila CSAS has high specificity for N-acetylneuraminic acid as a substrate. Furthermore, DmCSAS shows a steep temperature dependence of activity , which suggests that environmental temperatures can regulate Drosophila sialylation, thus modulating neural transmission.
Intriguingly, our genetic analyses also indicated that sialic acids may function as masking modifications that regulate interactions of N-glycans with some yet unidentified lectins. Our current experiments concentrate on elucidation of molecular and genetic mechanisms that control sialylation, as well as revealing its functional targets.
Publications
Akishina AA, Vorontsova JE, Cherezov RO, Mertsalov IB, Zatsepina OG, Slezinger MS, Panin VM, Petruk S, Enikolopov GN, Mazo A, Simonova OB, Kuzin BA (2017) Xenobiotic-induced activation of human aryl hydrocarbon receptor target genes in Drosophila is mediated by the epigenetic chromatin modifiers. Oncotarget. 8(61):102934-102947.
Mertsalov IB, Novikov, BN, Scott, H, Dangott, L, Panin, VM. (2016) Characterization of Drosophila CMP-sialic acid synthetase activity reveals unusual enzymatic properties. Biochem J. 473(13):1905-16.
Scott, H. and Panin, V.M. (2014) The role of protein N-glycosylation in neural transmission. Glycobiology, 24: 407-417.
Islam R, Nakamura M, Scott H, Repnikova E, Carnahan M, Pandey D, Caster C, Khan S, Zimmermann T, Zoran MJ, Panin VM (2013) The role of Drosophila cytidine monophosphate-sialic acid synthetase in the nervous system. J Neurosci 33:12306-12315.
Nakamura M, Pandey D, Panin VM (2012) Genetic interactions between Drosophila sialyltransferase and beta1,4-N-acetylgalactosaminyltransferase-A genes indicate their involvement in the same pathway. G3: Genes Genomes Genetics, 2(6): 653-656
Repnikova E, Koles K, Nakamura M, Pitts J,Li H, Ambavane A, Zoran MJ, Panin VM. (2010) Sialyltransferase regulates nervous system function in Drosophila. J Neurosci. 30(18): 6466-76 *Preview: J Neurosci. (2010) 30(18): i
Koles K, Repnikova E, Pavlova G, Korochkin LI, Panin VM. (2009) Sialylation in protostomes: a perspective from Drosophila genetics and biochemistry. Glycoconj J. 26(3):313-24
Koles, K., Lim, J.-M, Aoki, K., Porterfield, M.,Tiemeyer, M., Wells, L., and Panin, V.M. (2007) Identification of N-glycosylated glycoproteins from the central nervous system of Drosophila melanogaster. Glycobiology.17(12): 1388-1403.
North, S.J., Koles, K., Hembd, C., Morris, H.R., Dell, A., Panin, V.M., and Haslam, S.M. (2006) Glycomic studies of Drosophila melanogaster embryos. Glycoconjugate Journal 23: 345-354
Koles, K., Irvine, K.D, Panin, V.M. (2004) Functional characterization of Drosophila Sialyltransferase. J Biol Chem. 279: 4346-57.
Koles, K., Irvine, K.D, Panin, V.M. (2004) Functional characterization of DrosophilaSialyltransferase. J Biol Chem. 279: 4346-57.
